Long QT Syndrome
| Author(s) | J.S.S.G. de Jong, MD | |
| Moderator | P.G. Postema, MD | |
| Supervisor | ||
| some notes about authorship | ||
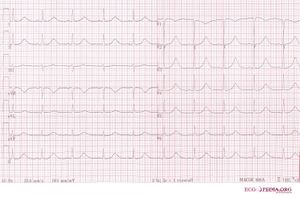
The Long QT Syndrome (LQTS) is characterized on the ECG by prolongation of the heart rate corrected QT interval. This was first recognized by dr. Jervell and dr. Lange-Nielsen in 1957. They described 4 children with a long QT interval which was accompanied by hearing deficits, sudden cardiac death and an autosomal recessive inheritance.[1]
The Long QT syndrome may be divided into two distinct forms: congenital Long QT syndrome and acquired Long QT syndrome. These forms may however overlap when QT prolongation due to medication occurs in a patient with congenital Long QT syndrome.
Diagnosis
- The diagnosis is by measurement of the heart rate-corrected QT interval on the ECG, which can be calculated with the QTc calculator.
- Sometimes the QT interval can be difficult to assess. Read the guidelines for measurement of difficult QT interval.
- A QTc of > 500ms in patients with Long QT Syndrome is associated with an increased risk for sudden death.[2]
- In patients suspected of LQTS (e.g. family members of known LQTS patients) a QTc > 430ms makes it likely that a LQTS gene defect is present.[3]
- Because the QTc can change with age, it is best to take the ECG with the longest QTc interval for risk stratification.[4]
Treatment[5]
- "Lifestyle modification":
- No competitive sports in all LQTS patients
- No swimming in LQT1 patients
- Avoid nightly noise in LQT2 patients (e.g. no alarm clock)
- Medication: beta-blockers. Beta-blockers even reduce the risk of sudden death in patients in whom a genetic defect has been found, but no QT prolongation is visible on the ECG.
- ICD implantation in combination with beta-blockers in LQTS patients with previous cardiac arrest or syncope or ventricular tachycardia while on beta-blockers.
Acquired LQTS
Acquired LQTS is most often caused by drugs that prolong the QT interval. Combined with risk factors (see table) the risk of Torsade de Pointes increases.
|
|
Congenital LQTS
:
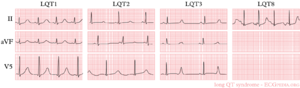
- LQT1 'early onset' broad based T wave
- LQT2 small late T wave
- LQT3 prolonged QT interval with 'late onset' T wave with a normal configuration
In congenital LQTS the ventricular repolarisation is prolonged. The prevalence is about 1:3000-5000.
More than 10 different types of congenital LQTS have been described. However, only LQTS 1-3 are relatively common.[5]
| Type | Chromosome | Gene | Protein | Ionchannel | Frequency[7] | SCD incidence[8] | Inheritance | ECG characteristics | Trigger | Eponyme | OMIM™ link |
| LQTS1 | 11p15 | KCNQ1 | KvLQT1 | Iks | ~50% | 0.30%/year | AD, AR | broad base 'early onset' T wave | exercise, especially swimming | JLN1 if homozygous, LQTS1 if heterozygous | 607542 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LQTS2 | 7q35 | KCNH2 | hERG | Ikr | 30-40% | 0.60%/year | AD | small late T wave | adrenergic triggers, especially nightly noise | JLN2 if homozygous, LQTS2 if heterozygous | 152427 |
| LQTS3 | 3p21 | SCN5A | NA channel | 5-10% | 0.56%/year | AD | 'Late onset' T wave with normal configuration | 600163 | |||
| LQTS4 | 4q25-q27 | ANK2 | Ankyrin B | INa,K | <1% | AD | 106410 | ||||
| LQTS5 | 21q22.1 | KCNE1 | minK | Iks | <1% | unknown | AD/AR | 176261 | |||
| LQTS6 | 21q22.1 | KCNE2 | MiRP1 | Ikr | <1% | unknown | AD | 603796 | |||
| LQTS7 = ATS1 | 17q23 | KCNJ2 | Kir 2.1 | IK1 | <1% | unknown | AD | Anderson-Tawil syndrome | 600681 | ||
| LQTS8 = TS1 | 12p13.3 | CACNA1C | Cav1.2 | ICa-L | <1% | unknown | alternating T waves | Timothy syndrome | 601005 | ||
| LQTS9 | 3p25.3 | CAV3 | Caveolin 3 | INa | unknown | 601253 | |||||
| LQTS10 | 11q23.3 | SCN4B | Nav1.5 b4 | 1 family | unknown | 608256 | |||||
| LQTS11 | 7q21-q22 | Akap9 | AKAP | Iks | 1 family | unknown | 611820 |
- LQTS
- Long QT syndrome
- JLN
- Jervell and Lange-Nielsen syndrome
- SCD
- Sudden Cardiac Death
Long before the genes involved were known, two syndromes associated with a prolonged QT interval on the ECG had been described.
- Anton Jervell and Fred Lange-Nielsen from Oslo described in 1957 an autosomaal recessive syndrome that was associated with QT interval prolongation, deafness and sudden death: the now called Jervell-Lange-Nielsen syndrome. [1]
- Romano-Ward syndrome is a long QT syndrome with normal auditory function and autosomal dominant inheritance.
- In a genotype–phenotype study by Moss et al. that studied type-1 LQTS, it was found that mutations located in the transmembrane portion of the ion channel protein and the degree of ion channel dysfunction caused by the mutations are important independent risk factors influencing the clinical course of this disorder.[9]
External links
Referenties
- JERVELL A and LANGE-NIELSEN F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J. 1957 Jul;54(1):59-68. DOI:10.1016/0002-8703(57)90079-0 |
- Hofman N, Wilde AA, Kääb S, van Langen IM, Tanck MW, Mannens MM, Hinterseer M, Beckmann BM, and Tan HL. Diagnostic criteria for congenital long QT syndrome in the era of molecular genetics: do we need a scoring system?. Eur Heart J. 2007 Mar;28(5):575-80. DOI:10.1093/eurheartj/ehl355 |
- Goldenberg I, Mathew J, Moss AJ, McNitt S, Peterson DR, Zareba W, Benhorin J, Zhang L, Vincent GM, Andrews ML, Robinson JL, and Morray B. Corrected QT variability in serial electrocardiograms in long QT syndrome: the importance of the maximum corrected QT for risk stratification. J Am Coll Cardiol. 2006 Sep 5;48(5):1047-52. DOI:10.1016/j.jacc.2006.06.033 |
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology/American Heart Association Task Force, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association, and Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006 Sep 5;114(10):e385-484. DOI:10.1161/CIRCULATIONAHA.106.178233 |
- Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med. 2008 Jan 10;358(2):169-76. DOI:10.1056/NEJMcp0706513 |
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004 Mar 4;350(10):1013-22. DOI:10.1056/NEJMra032426 |
- Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, and Cappelletti D. Risk stratification in the long-QT syndrome. N Engl J Med. 2003 May 8;348(19):1866-74. DOI:10.1056/NEJMoa022147 |
- Shah M, Akar FG, and Tomaselli GF. Molecular basis of arrhythmias. Circulation. 2005 Oct 18;112(16):2517-29. DOI:10.1161/CIRCULATIONAHA.104.494476 |
- Moss AJ, Shimizu W, Wilde AA, Towbin JA, Zareba W, Robinson JL, Qi M, Vincent GM, Ackerman MJ, Kaufman ES, Hofman N, Seth R, Kamakura S, Miyamoto Y, Goldenberg I, Andrews ML, and McNitt S. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007 May 15;115(19):2481-9. DOI:10.1161/CIRCULATIONAHA.106.665406 |
- Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, and Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001 Jan 2;103(1):89-95. DOI:10.1161/01.cir.103.1.89 |