Arrhythmogenic Right Ventricular Cardiomyopathy: Difference between revisions
Secretariat (talk | contribs) No edit summary |
mNo edit summary |
||
| (19 intermediate revisions by 4 users not shown) | |||
| Line 8: | Line 8: | ||
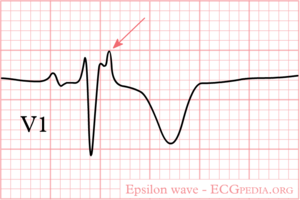
[[Image:epsilon_wave.png|thumb|ECG with an epsilon wave in V1]] | [[Image:epsilon_wave.png|thumb|ECG with an epsilon wave in V1]] | ||
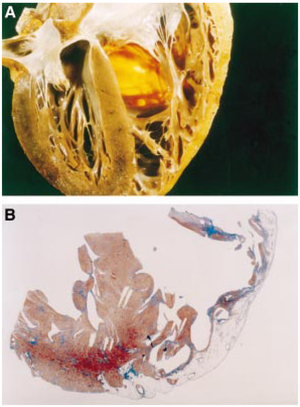
[[Image:arvdhart.png|thumb| A section throughout the heart of an ARVC patient. (A) Transmural fatty replacement of the right ventricular free wall. (B) Myocardial atrophy is confined to the right ventricle and substantially spares the interventricular septum as well as the left ventricular free wall. <cite>Corrado</cite> Reproduced with permission from BMJ Publishing Group Ltd. ]] | [[Image:arvdhart.png|thumb| A section throughout the heart of an ARVC patient. (A) Transmural fatty replacement of the right ventricular free wall. (B) Myocardial atrophy is confined to the right ventricle and substantially spares the interventricular septum as well as the left ventricular free wall. <cite>Corrado</cite> Reproduced with permission from BMJ Publishing Group Ltd. ]] | ||
'''Arrhythmogenic Right Ventricular Cardiomyopathy''', (ARVC, or ARVD: Arrhythmogenic Right Ventricular Disease) is characterized by fatty replacement and fibrosis of the heart. Most commonly the right ventricle apex and outflow tract are involved. However the left ventricle can be affected | '''Arrhythmogenic Right Ventricular Cardiomyopathy''', (ARVC, or ARVD: Arrhythmogenic Right Ventricular Disease) is characterized by fatty replacement and fibrosis of the heart. Most commonly the right ventricle apex and outflow tract are involved. However the left ventricle can be affected too.<cite>Corr</cite> | ||
As a result of the fatty replacement and fibrosis, ventricular arrhythmias are common in this disease and can lead to palpitations, syncope and sudden death. At more advanced ages right ventricular pump failure can occur. | As a result of the fatty replacement and fibrosis, ventricular arrhythmias are common in this disease and can lead to palpitations, syncope and sudden death. At more advanced ages right ventricular pump failure can occur. | ||
| Line 14: | Line 14: | ||
The diagnosis is based on major and minor criteria, as published by the European Society of Cardiology.<cite>McKenna1994</cite> | The diagnosis is based on major and minor criteria, as published by the European Society of Cardiology.<cite>McKenna1994</cite> | ||
ARVC is a progressive disease. The '''incidence''' is estimated to be 1:3.000-1:10.000. Manifestations are usually seen in teenagers. Although the diagnosis is more often made in athletes, sports are not thought to have a causative relationship with the disease. ARVD can occur in families; more than 9 different | ARVC is a progressive disease. The '''incidence''' is estimated to be 1:3.000-1:10.000. Manifestations are usually seen in teenagers. Although the diagnosis is more often made in athletes, sports are not thought to have a causative relationship with the disease. ARVD can occur in families; more than 9 different mutations have been described, most often with autosomal dominant inheritance. | ||
One unique form of ARVD, called Naxos disease (after the Greek island where it was first diagnosed), has an autosomal recessive pattern of inheritance. | One unique form of ARVD, called Naxos disease (after the Greek island where it was first diagnosed), has an autosomal recessive pattern of inheritance. | ||
'''Diagnosis''' ARVC is a difficult diagnosis to make. Therefore, the European Society of Cardiology has created a list of diagnostic criteria for the diagnosis of ARVC<cite>#McKenna1994</cite> (see table). | '''Diagnosis''' ARVC is a difficult diagnosis to make. Therefore, the European Society of Cardiology has created a list of diagnostic criteria for the diagnosis of ARVC<cite>#McKenna1994</cite> (see table). An [http://www.arvc.ca/pdg/public.php?rep=arvc_cri online calculator] can help in assessing the risk in an individual patient. In 2009 these criteria were updated<cite>cox</cite><cite>Crit2010</cite>, see the table below. | ||
'''Treatment''' focuses on avoiding complications.<cite>ACC2006</cite> | '''Treatment''' focuses on avoiding complications.<cite>ACC2006</cite> | ||
| Line 51: | Line 27: | ||
*[[ICD]] implantation can be considered for the prevention of sudden cardiac death in patients with ARVC with extensive disease, including those with left ventricular involvement, 1 or more affected family member with SCD (Sudden Cardiac Death), or undiagnosed syncope when [[Ventricular Tachycardia|ventricular tachycardia]] or [[Ventricular Fibrillation|ventricular Fibrillation]] has not been excluded as the cause of syncope, who are receiving chronic optimal medical therapy, and who have reasonable expectation of survival with a good functional status for more than 1 y. | *[[ICD]] implantation can be considered for the prevention of sudden cardiac death in patients with ARVC with extensive disease, including those with left ventricular involvement, 1 or more affected family member with SCD (Sudden Cardiac Death), or undiagnosed syncope when [[Ventricular Tachycardia|ventricular tachycardia]] or [[Ventricular Fibrillation|ventricular Fibrillation]] has not been excluded as the cause of syncope, who are receiving chronic optimal medical therapy, and who have reasonable expectation of survival with a good functional status for more than 1 y. | ||
*Radiofrequency [[ablation]] can be useful as adjunctive therapy in management of patients with ARVC with recurrent [[Ventricular Tachycardia|ventricular tachycardia]], despite optimal antiarrhythmic drug therapy. | *Radiofrequency [[ablation]] can be useful as adjunctive therapy in management of patients with ARVC with recurrent [[Ventricular Tachycardia|ventricular tachycardia]], despite optimal antiarrhythmic drug therapy. | ||
== ECG in ARVD== | |||
On [http://www.arvd.org ARVD.org] the latest ECG criteria for ARVD are updated. To enhance detection of ECG abnormalities specific settings to record an ARVD ECG can be used: [http://arvd.org/ECG.pdf ECG settings to enhance recording of Epsilon waves]. | |||
In patients suspected to have ARVD both a normal 12 lead ECG and a Signal Averaged ECG ([[SAECG]]) are useful. | |||
<gallery> | <gallery> | ||
Image:arvd_ecg1.png | Image:arvd_ecg1.png| a patient with ARVD | ||
Image:arvd_ecg2.png | Image:arvd_ecg2.png | ||
Image:arvd_ecg3.png | Image:arvd_ecg3.png | ||
</gallery> | </gallery> | ||
{| class="wikitable" | == Revised Task Force Criteria ARVD / ARVC== | ||
|+ | {| class="wikitable" style="width:700px" | ||
! | |+ The Revised Task Force Criteria for ARVD / ARVC | ||
! colspan="2" style="width:600px" | Revised Task Force Criteria | |||
|- | |- | ||
| colspan=" | | colspan="2" | '''I. Global or regional dysfunction and structural alterations<sup>∗</sup>''' | ||
|- | |- | ||
| | | style="width:300px" | '''Major''' | ||
| style="width:300px" | '''Minor''' | |||
|- | |- | ||
| valign="top" style="padding-left:24px" | | |||
| valign="top" | |||
<strong>By 2D echo:</strong> | <strong>By 2D echo:</strong> | ||
* Regional RV akinesia, dyskinesia, or aneurysm | * Regional RV akinesia, dyskinesia, or aneurysm | ||
| Line 87: | Line 62: | ||
<strong>By RV angiography:</strong> | <strong>By RV angiography:</strong> | ||
* Regional RV akinesia, dyskinesia, or aneurysm | * Regional RV akinesia, dyskinesia, or aneurysm | ||
| valign="top" | | |||
<strong>By 2D echo:</strong> | |||
* Regional RV akinesia or dyskinesia | |||
* <em>and</em> 1 of the following (end diastole): | |||
** PLAX RVOT ≥29 to <32 mm (corrected for body size [PLAX/BSA] ≥16 to <19 mm/m<sup>2</sup>) | |||
** PSAX RVOT ≥32 to <36 mm (corrected for body size [PSAX/BSA] ≥18 to <21 mm/m<sup>2</sup>) | |||
** <em>or</em> fractional area change >33% to ≤40% | |||
<strong>By MRI:</strong> | |||
* Regional RV akinesia or dyskinesia or dyssynchronous RV contraction | |||
* <em>and</em> 1 of the following: | |||
** Ratio of RV end-diastolic volume to BSA ≥100 to <110 mL/m<sup>2</sup> (male) or ≥90 to <100 mL/m<sup>2</sup> (female) | |||
** <em>or</em> RV ejection fraction >40% to ≤45% | |||
|- | |||
| colspan="2" | '''II. Tissue characterization of wall''' | |||
|- | |||
| '''Major''' | |||
| '''Minor''' | |||
|- | |||
| valign="top" | | |||
* Residual myocytes <60% by morphometric analysis (or <50% if estimated), with fibrous replacement of the RV free wall myocardium in ≥1 sample, with or without fatty replacement of tissue on endomyocardial biopsy | |||
| valign="top" | | |||
* Residual myocytes 60% to 75% by morphometric analysis (or 50% to 65% if estimated), with fibrous replacement of the RV free wall myocardium in ≥1 sample, with or without fatty replacement of tissue on endomyocardial biopsy | |||
|- | |||
| colspan="2" | '''III. Repolarization abnormalities''' | |||
|- | |||
| '''Major''' | |||
| '''Minor''' | |||
|- | |||
| valign="top" | | |||
* Inverted T waves in right precordial leads (V<sub>1</sub>, V<sub>2</sub>, and V<sub>3</sub>) or beyond in individuals >14 years of age (in the absence of complete right bundle-branch block QRS ≥120 ms) | |||
| valign="top" | | |||
* Inverted T waves in leads V<sub>1</sub> and V<sub>2</sub> in individuals >14 years of age (in the absence of complete right bundle-branch block) or in V<sub>4</sub>, V<sub>5</sub>, or V<sub>6</sub> | |||
* Inverted T waves in leads V<sub>1</sub>, V<sub>2</sub>, V<sub>3</sub>, and V<sub>4</sub> in individuals >14 years of age in the presence of complete right bundle-branch block | |||
|- | |||
| colspan="2" | '''IV. Depolarization/conduction abnormalities''' | |||
|- | |||
| '''Major''' | |||
| '''Minor''' | |||
|- | |||
| valign="top" | | |||
* Epsilon wave (reproducible low-amplitude signals between end of QRS complex to onset of the T wave) in the right precordial leads (V<sub>1</sub> to V<sub>3</sub>) | |||
| valign="top | | |||
* Late potentials by SAECG in ≥1 of 3 parameters in the absence of a QRS duration of ≥110 ms on the standard ECG | |||
* Filtered QRS duration (fQRS) ≥114 ms | |||
* Duration of terminal QRS <40 <em>µ</em>V (low-amplitude signal duration) ≥38 ms | |||
* Root-mean-square voltage of terminal 40 ms ≤20 <em>µ</em>V | |||
* Terminal activation duration of QRS ≥55 ms measured from the nadir of the S wave to the end of the QRS, including R´, in V<sub>1</sub>, V<sub>2</sub>, or V<sub>3</sub>, in the absence of complete right bundle-branch block | |||
|- | |||
| colspan="2" | '''V. Arrhythmias''' | |||
|- | |||
| '''Major''' | |||
| '''Minor''' | |||
|- | |||
| valign="top" | | |||
* Nonsustained or sustained ventricular tachycardia of left bundle-branch morphology with superior axis (negative or indeterminate QRS in leads II, III, and aVF and positive in lead aVL) | |||
| valign="top | | |||
* Nonsustained or sustained ventricular tachycardia of RV outflow configuration, left bundle-branch block morphology with inferior axis (positive QRS in leads II, III, and aVF and negative in lead aVL) or of unknown axis | |||
* >500 ventricular extrasystoles per 24 hours (Holter) | |||
|- | |||
| colspan="2" | '''VI. Family history''' | |||
|- | |||
| '''Major''' | |||
| '''Minor''' | |||
|- | |||
| valign="top" | | |||
* ARVC/D confirmed in a first-degree relative who meets current Task Force criteria | |||
* ARVC/D confirmed pathologically at autopsy or surgery in a first-degree relative | |||
* Identification of a pathogenic mutation<sup>†</sup> categorized as associated or probably associated with ARVC/D in the patient under evaluation | |||
| valign="top | | |||
* History of ARVC/D in a first-degree relative in whom it is not possible or practical to determine whether the family member meets current Task Force criteria | |||
* Premature sudden death (<35 years of age) due to suspected ARVC/D in a first-degree relative | |||
* ARVC/D confirmed pathologically or by current Task Force Criteria in second-degree relative | |||
|- | |||
| colspan="2" | | |||
<ul> | |||
<li>PLAX indicates parasternal long-axis view; RVOT, RV outflow tract; BSA, body surface area; PSAX, parasternal short-axis view; aVF, augmented voltage unipolar left foot lead; and aVL, augmented voltage unipolar left arm lead.</li> | |||
<li>Diagnostic terminology for original criteria: This diagnosis is fulfilled by the presence of 2 major, or 1 major plus 2 minor criteria or 4 minor criteria from different groups. Diagnostic terminology for revised criteria: definite diagnosis: 2 major or 1 major and 2 minor criteria or 4 minor from different categories; borderline: 1 major and 1 minor or 3 minor criteria from different categories; possible: 1 major or 2 minor criteria from different categories.</li> | |||
<li><sup>∗</sup> Hypokinesis is not included in this or subsequent definitions of RV regional wall motion abnormalities for the proposed modified criteria.</li> | |||
<li><sup>†</sup> A pathogenic mutation is a DNA alteration associated with ARVC/D that alters or is expected to alter the encoded protein, is unobserved or rare in a large non–ARVC/D control population, and either alters or is predicted to alter the structure or function of the protein or has demonstrated linkage to the disease phenotype in a conclusive pedigree. E.g.: in TMEM43, DSP, PKP2, DSG2, DSC2, JUP. </li> | |||
</ul> | |||
|} | |} | ||
| Line 95: | Line 150: | ||
#ACC2006 pmid=16949478 | #ACC2006 pmid=16949478 | ||
#Corr pmid=19366719 | #Corr pmid=19366719 | ||
# | #cox pmid=20215590 | ||
#Crit2010 pmid=20172912 | |||
</biblio> | </biblio> | ||
Latest revision as of 13:20, 5 May 2013
| Author(s) | J.S.S.G. de Jong, MD | |
| Moderator | J.S.S.G. de Jong, MD | |
| Supervisor | ||
| some notes about authorship | ||
Arrhythmogenic Right Ventricular Cardiomyopathy, (ARVC, or ARVD: Arrhythmogenic Right Ventricular Disease) is characterized by fatty replacement and fibrosis of the heart. Most commonly the right ventricle apex and outflow tract are involved. However the left ventricle can be affected too.[2]
As a result of the fatty replacement and fibrosis, ventricular arrhythmias are common in this disease and can lead to palpitations, syncope and sudden death. At more advanced ages right ventricular pump failure can occur.
The diagnosis is based on major and minor criteria, as published by the European Society of Cardiology.[3]
ARVC is a progressive disease. The incidence is estimated to be 1:3.000-1:10.000. Manifestations are usually seen in teenagers. Although the diagnosis is more often made in athletes, sports are not thought to have a causative relationship with the disease. ARVD can occur in families; more than 9 different mutations have been described, most often with autosomal dominant inheritance.
One unique form of ARVD, called Naxos disease (after the Greek island where it was first diagnosed), has an autosomal recessive pattern of inheritance.
Diagnosis ARVC is a difficult diagnosis to make. Therefore, the European Society of Cardiology has created a list of diagnostic criteria for the diagnosis of ARVC[3] (see table). An online calculator can help in assessing the risk in an individual patient. In 2009 these criteria were updated[4][5], see the table below.
Treatment focuses on avoiding complications.[6]
- Medication:
- Anti-arrhythmics: Sotalol better than Amiodarone.
- ACE-inhibitors to prevent cardiac remodelling
- ICD implantation is recommended for the prevention of sudden cardiac death in patients with ARVC with documented sustained VT or VF who are receiving chronic optimal medical therapy.
- ICD implantation can be considered for the prevention of sudden cardiac death in patients with ARVC with extensive disease, including those with left ventricular involvement, 1 or more affected family member with SCD (Sudden Cardiac Death), or undiagnosed syncope when ventricular tachycardia or ventricular Fibrillation has not been excluded as the cause of syncope, who are receiving chronic optimal medical therapy, and who have reasonable expectation of survival with a good functional status for more than 1 y.
- Radiofrequency ablation can be useful as adjunctive therapy in management of patients with ARVC with recurrent ventricular tachycardia, despite optimal antiarrhythmic drug therapy.
ECG in ARVD
On ARVD.org the latest ECG criteria for ARVD are updated. To enhance detection of ECG abnormalities specific settings to record an ARVD ECG can be used: ECG settings to enhance recording of Epsilon waves.
In patients suspected to have ARVD both a normal 12 lead ECG and a Signal Averaged ECG (SAECG) are useful.
Revised Task Force Criteria ARVD / ARVC
| Revised Task Force Criteria | |
|---|---|
| I. Global or regional dysfunction and structural alterations∗ | |
| Major | Minor |
|
By 2D echo:
By MRI:
By RV angiography:
|
By 2D echo:
By MRI:
|
| II. Tissue characterization of wall | |
| Major | Minor |
|
|
| III. Repolarization abnormalities | |
| Major | Minor |
|
|
| IV. Depolarization/conduction abnormalities | |
| Major | Minor |
|
|
| V. Arrhythmias | |
| Major | Minor |
|
|
| VI. Family history | |
| Major | Minor |
|
|
| |
References
- Corrado D, Basso C, and Thiene G. Arrhythmogenic right ventricular cardiomyopathy: diagnosis, prognosis, and treatment. Heart. 2000 May;83(5):588-95. DOI:10.1136/heart.83.5.588 |
- Corrado D, Basso C, and Thiene G. Arrhythmogenic right ventricular cardiomyopathy: an update. Heart. 2009 May;95(9):766-73. DOI:10.1136/hrt.2008.149823 |
- McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, and Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994 Mar;71(3):215-8. DOI:10.1136/hrt.71.3.215 |
- Cox MG, van der Smagt JJ, Noorman M, Wiesfeld AC, Volders PG, van Langen IM, Atsma DE, Dooijes D, Houweling AC, Loh P, Jordaens L, Arens Y, Cramer MJ, Doevendans PA, van Tintelen JP, Wilde AA, and Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy diagnostic task force criteria: impact of new task force criteria. Circ Arrhythm Electrophysiol. 2010 Apr;3(2):126-33. DOI:10.1161/CIRCEP.109.927202 |
- Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, and Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010 Apr;31(7):806-14. DOI:10.1093/eurheartj/ehq025 |
- European Heart Rhythm Association, Heart Rhythm Society, Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology, American Heart Association Task Force, and European Society of Cardiology Committee for Practice Guidelines. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol. 2006 Sep 5;48(5):e247-346. DOI:10.1016/j.jacc.2006.07.010 |